Easy and Affordable UDI Management
The Medical Device Regulation (MDR) is considered one of the most complex regulations worldwide. Therefore, medical technology companies need a validated, efficient, and practical system to manage the challenges of UDI management with ease, flexibility, and cost-effectiveness and report them to EUDAMED without any hurdles.
Our software solution, mytracekey UDI Manager, allows any medical technology company to comply with regulatory requirements. Regardless of the company’s size, UDI management with our software is not only simple and cost-effective but also enhances the quality of your master data management and compliance.
Overview of the Available Packages
We offer the mytracekey UDI Manager in various packages to best suit your needs.
Starter Package: mytracekey UDI Manager BASIC, for a few UDIs or as a trial version.
Full Version: mytracekey UDI Manager FULL is equipped with all the extras, such as an Audit Trail, Task Manager, and validation documents, yet it is still compact.
Enterprise: mytracekey MedTech, for high UDI volumes with additional service and support for your quality management.
If you’re uncertain about which package best suits your needs, we invite you to explore our guide for detailed information on each option.
5 Reasons to Use our UDI Manager
1. Simple Master Data Management
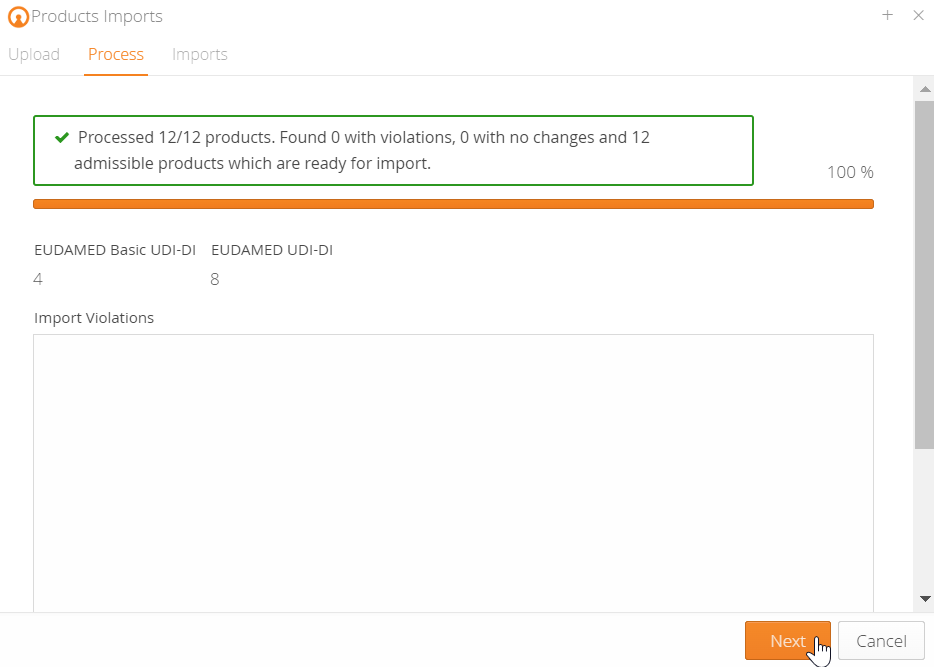
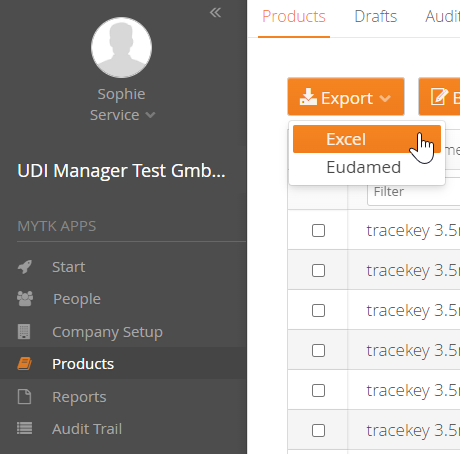
You can easily import your UDI data into the mytracekey UDI Manager using an Excel spreadsheet. After a successful data check, you can create the files for EUDAMED via the XML bulk upload function, or use the M2M interface in the FULL version for direct upload from the system.
2. Flexible Subscription
Benefit from the flexibility of our subscription model: you decide how long you want to use the UDI Manager. Our monthly contract term makes it possible!
3. Pay-Per-Use
Depending on the product variant, a fixed number of UDIs is included in the package price. If you need more, a fixed amount is charged for each additional UDI recorded (see table). At the end of the month, you will receive an invoice consisting of the package price and additional UDIs.
4. Everything Considered
Our UDI Manager enables you to validate your UDI data according to EUDAMED guidelines. It supports all product templates, such as MDR, MDD, IVDR, IVDD, and AIMDD.
5. Long-Term Benefits
Thanks to our established Life Science Cloud, we can offer the UDI Manager particularly cost-effectively. This allows you to take advantage of centralized UDI management in the long term – whether to be well-prepared for the next audit, to have your data including change history centrally available, or simply to avoid the tedious maintenance of Excel spreadsheets.
Do you still have some questions? Schedule an non-binding demo with our Sales Manager or contact us.