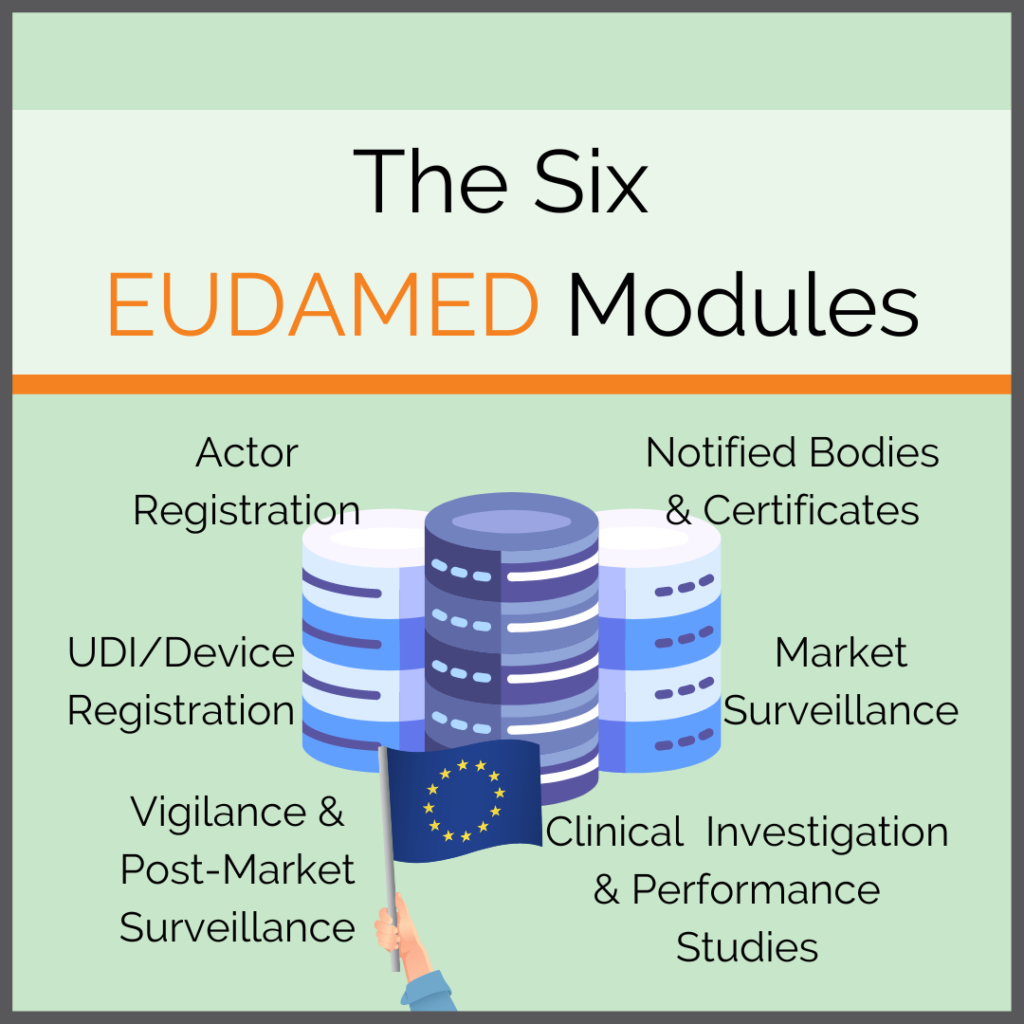
In the context of the MDR, UDIs are an essential topic. After all, they not only have to be applied to the product and label itself. They also serve as a key for the EUDAMED and are part of the documentation. We have summarised all information on the different UDI types for you and defined […]
New Whitepaper: 7 Steps to UDI Compliance