UDI Product Data Management for Medical Devices
Enterprise Solution for Big UDI Volumes
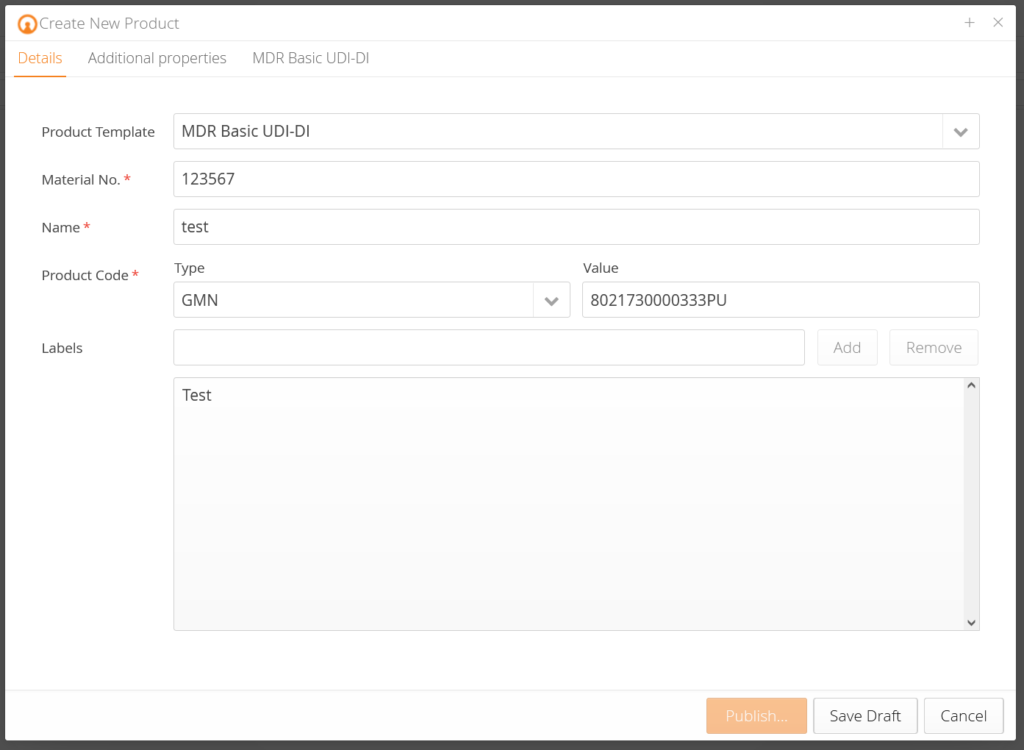
Regulations, such as the EU-MDR, pose complex challenges for medical technology companies. mytracekey MedTech is a straightforward, practical software solution (SaaS) that supports medical technology companies at the highest level in UDI management for EUDAMED. It is fully validated and has a direct interface to EUDAMED (M2M).
Optimize Your UDI Management with our Enterprise Solution
Medical device manufacturers with high UDI volumes benefit greatly from our advanced enterprise solution. Manually entering and updating hundreds or even thousands of UDIs is a significant challenge, even for large companies. The associated costs and time investment are substantial hurdles, and manual entry carries a high risk of errors.
Our solution offers you:
- Efficiency: Automate UDI management to save valuable time and resources.
- Security: Minimize errors through reliable and precise data processing, along with a comprehensive validation package, test, and production system.
- Experience: Benefit from a tool that has proven itself in practice and is continuously being developed.
Choose a solution that supports you, allowing you to focus on your core business.
Our enterprise solution, mytracekey MedTech, offers you a customized service package, including extensive support. This also includes personalized onboarding and user training.
Benefits
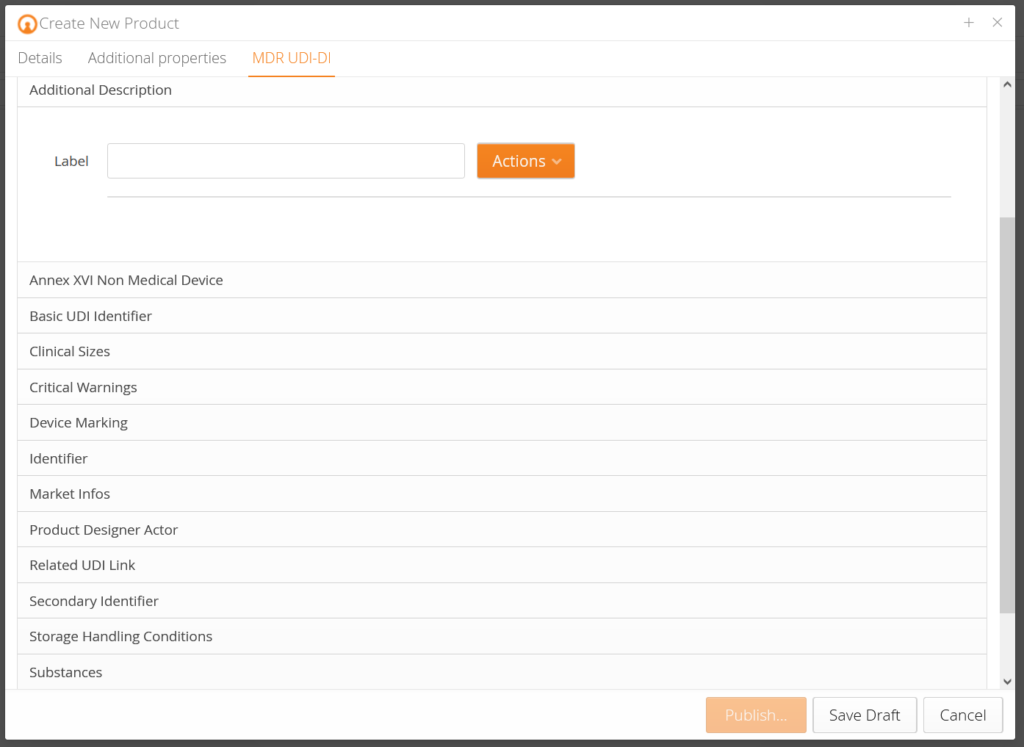
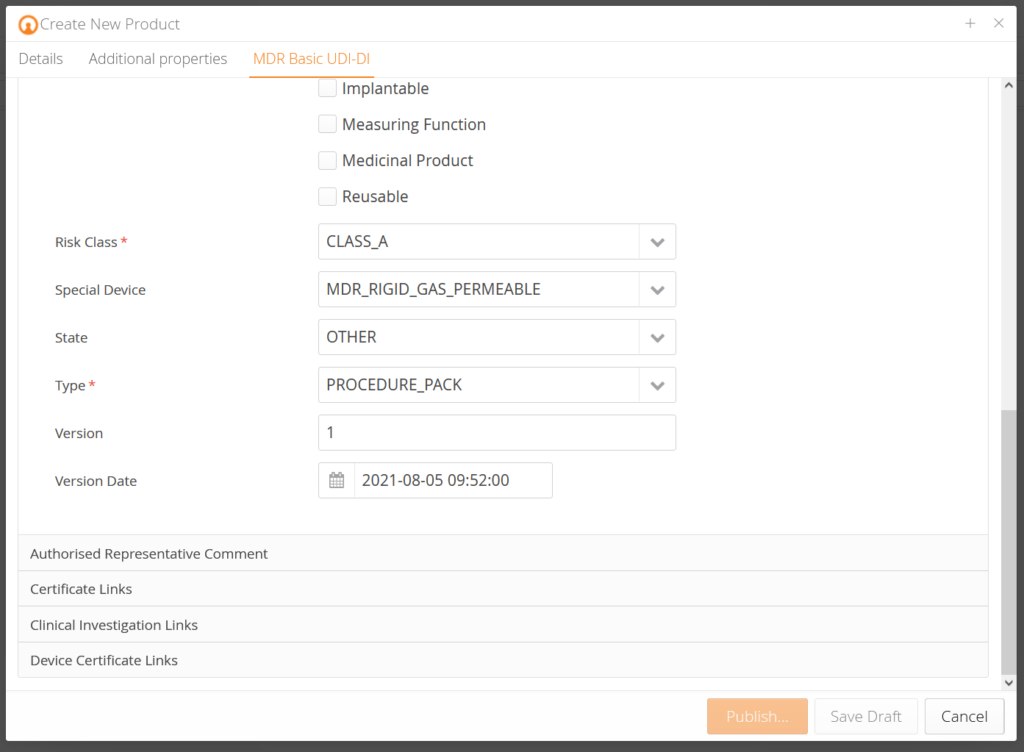
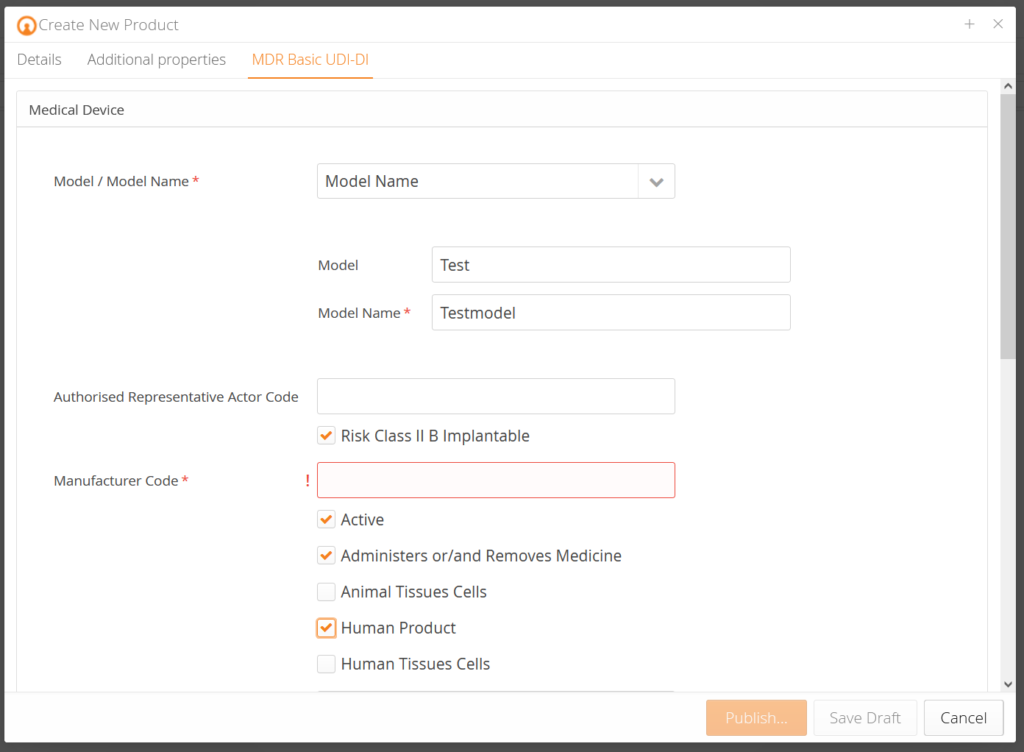
- Product Templates Included (MDD, MDR, IVDR, IVDD, AIMDD)
- Accurate and complete data thanks to EUDAMED Business Rules Check
- Easy data import via Excel
- Convenient EUDAMED reporting (M2M interface)
- Clear update process for existing data in EUDAMED
- Package of test and production environments provides extra security
- Better overview of upcoming reporting tasks through the Task Manager
- Quality management benefits from the audit trail
- No unnecessary product recalls due to incorrect or inconsistent data
- Comprehensive validation package facilitates quality management
- Redundant GxP cloud infrastructure
- Regular software updates
- No requirements for IT infrastructure
With mytracekey MedTech, we have developed an enterprise solution tailored for medical technology companies with a large UDI volume (8,000+) or high support needs. Companies with smaller UDI volumes and those preferring a self-service option can easily book our mytracekey UDI Manager online.
6 Reasons Why to Use mytracekey MedTech
Data Quality Requirements: Our solution pre-validates your data and maps product hierarchies, ensuring your product data is MDR-compliant from the start. This prevents unnecessary product recalls due to incorrect or inconsistent data.
Reporting to Authorities: Reporting to authorities is part of our daily routine. Whether through automated interfaces or manual workflows, our system and team know how to handle it.
Easy to Use: The clearly designed software offers a practical user experience thanks to the user manual and field-specific guidance. Additionally, you benefit from effective onboarding training.
Supply Chain Integration: Our solution acts as a central system where business partners can be integrated if needed, ensuring clear and documented data exchange.
International Regulations: We understand that a system must be future-proof by being adaptable to future requirements. Therefore, we also consider other international regulations.
Close to the Customer: We provide personal and approachable support from the beginning. Our modular support offerings are tailored to customer needs. We are committed to supporting our customers in the best possible way.
We Support your UDI Management

Download our white paper “7 Steps to UDI Compliance”. Download Whitepaper MDR-UDI Compliance
-> Get an overview of the EUDAMED modules
-> Can you meet the MDR requirements with your ERP system?
-> We have compiled helpful information material on the MDR and IVDR for you