Easy and Affordable UDI Management
The Medical Device Regulation (MDR) is considered one of the most complex regulations worldwide. Therefore, medical technology companies need a validated, efficient, and practical system to manage the challenges of UDI management with ease, flexibility, and cost-effectiveness and report them to EUDAMED without any hurdles.
Our software solution, mytracekey UDI Manager, allows any medical technology company to comply with regulatory requirements. Regardless of the company’s size, UDI management with our software is not only simple and cost-effective but also enhances the quality of your master data management and compliance.
Efficiency: Automate UDI management to save valuable time and resources.
Security: Minimize errors through reliable and precise data processing, along with a comprehensive validation package, test, and production system.
Experience: Benefit from a tool that has proven itself in practice and is continuously being developed.
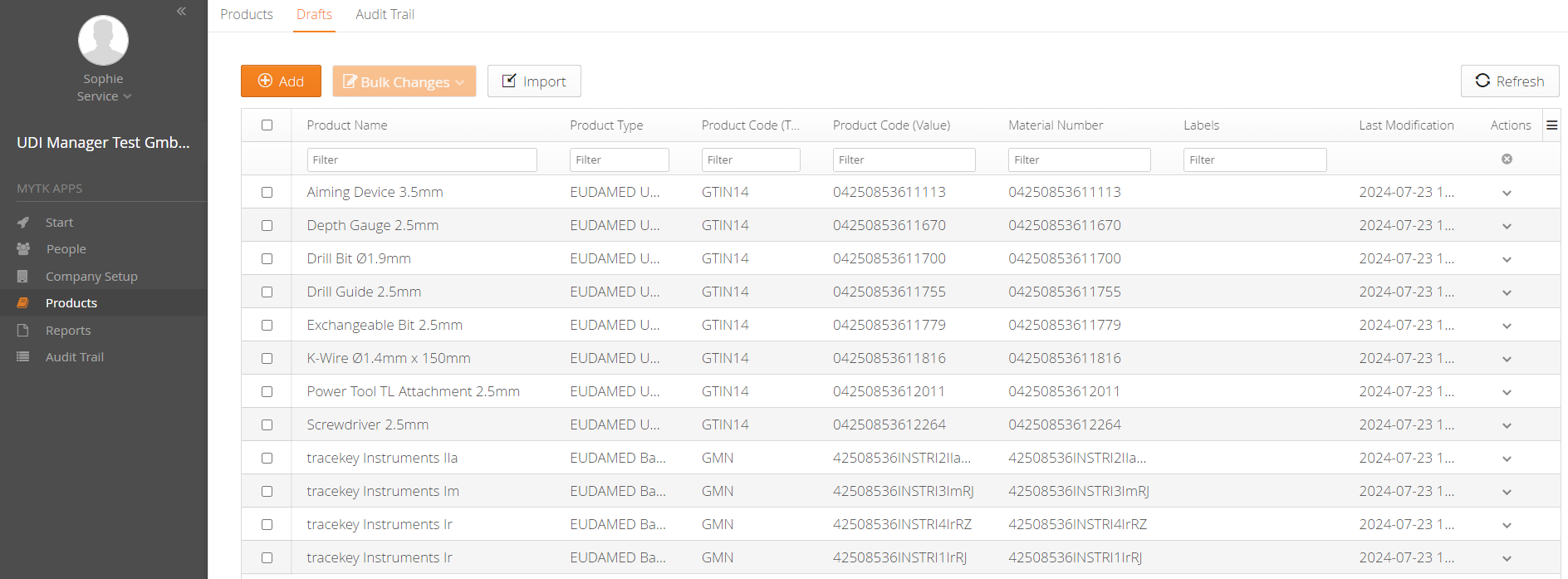
UDI Management Software
- Accurate and complete data thanks to EUDAMED Business Rules Check
- Convenient EUDAMED reporting (M2M interface)
- Clear update process for existing data in EUDAMED
- Better overview of upcoming reporting tasks through the Task Manager
- No unnecessary product recalls due to incorrect or inconsistent data
- Redundant GxP cloud infrastructure
- Regular software updates
- No requirements for IT infrastructure
Overview of the Available Packages
We offer the mytracekey UDI Manager in various packages to best suit your needs.
Free Version: mytracekey UDI Manager FREE, to get to know the software with limited features. You can find this version directly in our shop.
Starter Package: mytracekey UDI Manager BASIC, for a few UDIs, particularly flexible thanks to monthly contract terms.
Full Version: mytracekey UDI Manager FULL is equipped with all the extras, such as an Audit Trail, Task Manager, and validation documents, yet it is still compact.
Premium Version: mytracekey UDI Manager PREMIUM, manage three SRNs at once, with an integrated M2M connection.
Enterprise: mytracekey MedTech, for high UDI volumes with additional service and support for your quality management.
If you’re uncertain about which package best suits your needs, we invite you to explore our guide for detailed information on each option.
5 Reasons to Use our UDI Manager
1. Simple Master Data Management
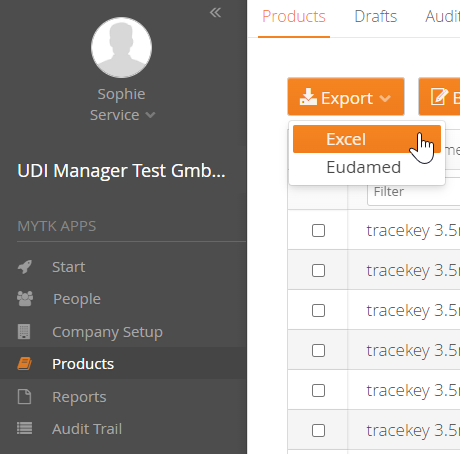
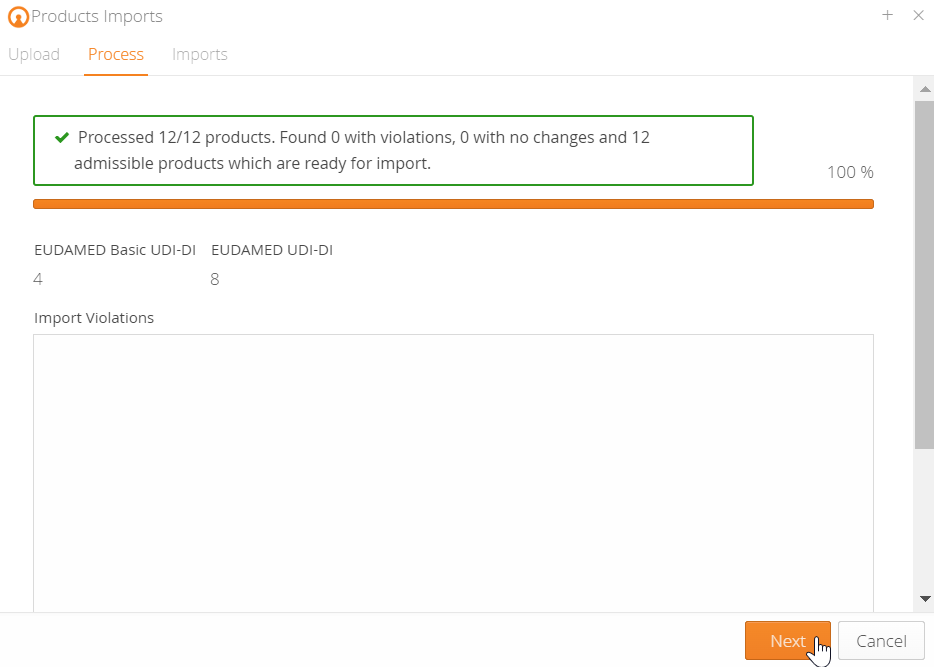
You can easily import your UDI data into the mytracekey UDI Manager using an Excel spreadsheet. After a successful data check, you can create the files for EUDAMED via the XML bulk upload function, or use the M2M interface in the FULL version for direct upload from the system.
2. Flexible Subscription
Benefit from the flexibility of our subscription model: you decide how long you want to use the UDI Manager. Our monthly contract term makes it possible!
3. Pay-Per-Use
Depending on the product variant, a fixed number of UDIs is included in the package price. If you need more, a fixed amount is charged for each additional UDI recorded (see table). At the end of the month, you will receive an invoice consisting of the package price and additional UDIs.
4. Everything Considered
Our UDI Manager enables you to validate your UDI data according to EUDAMED guidelines. It supports all product templates, such as MDR, MDD, IVDR, IVDD, and AIMDD.
5. Long-Term Benefits
Thanks to our established Healthcare cloud, we can offer the UDI Manager particularly cost-effectively. This allows you to take advantage of centralized UDI management in the long term – whether to be well-prepared for the next audit, to have your data including change history centrally available, or simply to avoid the tedious maintenance of Excel spreadsheets.
Do you still have questions and want to get a better overview? Just book a non-binding demo with us.
MedTech News

EUDAMED & UDI FAQ – The Most Important Questions on MDR, IVDR and Legacy Devices
In our webinars on EUDAMED and UDI, we receive many questions about legacy devices, the M2M interface, XML bulk upload, timelines, and responsibilities. [...] [...]Read More... from EUDAMED & UDI FAQ – The Most Important Questions on MDR, IVDR and Legacy Devices